While most people associate plastics with consumer items such as plastic food packaging or personal care products, they play a vital role in the medical field. Like “life or death” vital.
But plastics (also known as polymers) used in the medical device industry must meet higher standards than plastics used in other applications. The properties of these so-called “medical grade plastics” share common characteristics:
- they must be manufactured under a physician’s license to pass verification and validation requirements of governmental regulators
- they must offer biocompatibility. (More on that later.)
- they must be temperature, impact and corrosion resistant, enabling them to withstand high wear and repeated sterilization cycles
Common plastics utilized in the manufacturing of medical plastic products include polycarbonate, polypropylene, and polyethylene. In many cases, custom polymers are developed to meet specific plastic medical device applications. All of theses plastics are known as “thermoplastics,” which means they can be reheated and remolded repeatedly without irreversible degradation. Because the changes to thermoplastics which occur in plastic injection molding are physical, not chemical, devices made of thermoplastics can be reused and recycled.
When heated during the plastic injection molding process, thermoplastics liquefy, become easily moldable and solidify into a durable product after cooling. This makes thermoplastics ideal for custom medical device plastic injection molding.
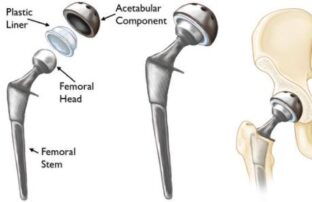
For example, many hip replacement implants, and nearly all total knee replacement implants, feature ultra-high molecular-weight polyethylene (UHMWPE). This plastic is necessary to provide cushioning and flexibility. Polyethylene is the most common plastic used in implantable devices because it is a porous synthetic polymer that is biologically inert. “Bioinert” materials are ones which don’t initiate a response or interact when introduced to biological tissue. In other words, introducing that particular material to the body won’t cause a reaction with the patient’s own body.
Polyethylene meets that definition, in spades. However, some polyethylene implants are “more equal” than others. A long-term follow-up study confirmed that hip implants which contain crossed-linked polyethylene (XLPE) substantially lowered the risk of a patient requiring revision surgery after a total hip replacement when compared to implants that contain conventional polyethylene (CPE) components. Cross-linked polyethylene (XLPE) foam is also the material of choice for plastic medical device packaging.
There’s no debate: plastics are the right prescription for medical parts and devices where failure is not an option.